I am sure that many readers have already concluded that I do not understand the role of sex in either organic or biotic evolution. At least I can claim, on the basis of the conflicting views in the recent literature, the consolation of abundant company.
– George C. Williams, Sex and Evolution, 1975
What’s sex all about? This question has been exercising biologists since well before Williams’s time, but in the 1970’s, with the rise of ‘gene-centrism’ and the related controversy over group selection, a succession of prominent authors grappled with the problem, trying to fit it with current evolutionary theory to no-one’s particular satisfaction. Males were deemed an impediment to a female’s efforts to maximise her reproductive output, time wasted on these feckless types resulting in her only passing on 50% of her genes per offspring. From the perspective of a ‘selfish gene’, meanwhile, getting into every offspring seems a preferable fate to only getting into half of them. On the basis of these apparent large costs, a cryptic offsetting benefit of corresponding magnitude was assumed. Like Godot, it is yet to appear.
Yet sex is widespread. All eukaryotes either do it now, or possess tell-tale signs that their recent ancestors did. Given that it appears costly to individuals, and genes, how did it evolve and why does it persist?
Terminology.
In asexual reproduction the entire genetic complement of cells or individuals – all of their chromosomes – is duplicated during mitosis. Two new cells are produced per complete division, giving exponential increase where unrestrained.
Mitosis occurs in sexual lineages too, but the defining characteristic of eukaryotic sexual reproduction is a cyclic alternation of ‘ploidy’, or chromosome number, interrupting the mitotic series. A haploid cell has one set of chromosomes, a diploid two, and so on, and in sex, two haploid gametes merge to form a diploid zygote in which each chromosome has a similar partner – a homologue – in the other haploid set.
At some future point, the reduction division of meiosis generates more haploid sets from the diploid, by pairing then segregating these homologous copies into new haploid cells. Additionally, during haploid separation there may be reciprocal swap of entire chromosomes or segments: recombination. By this process, previously linked genes end up in different individuals, while previously separate genes end up linked.
While in the diploid state, at any segment held in common between a pair of homologous chromosomes, the genetic sequence may be identical (homozygous) or not (heterozygous). Where heterozygous, the genome has two different alleles (sequence variants) at that locus, one of which may be dominant (expressed) and the other recessive (suppressed).
The problem
Consider an organism … with equal numbers of males and females … In females, a gene A suppresses meiosis, and causes the production of diploid eggs that develop without fertilization into females genetically identical to the parent. … when rare, such a gene would double in frequency in each generation. This result has been expressed by saying that there is a ‘twofold cost of sex’, arising from the needless production of males. It is clearer, however, to take a ‘gene’s eye view’: a gene A that suppresses meiosis is certain to be transmitted to all the eggs produced by a female, whereas a gene a that permits meiosis is transmitted to only half.
John Maynard Smith, Evolutionary Genetics 2nd Ed 1998.
The issue tends to be framed in population-genetic terms, by reduction to mathematical abstractions or computer models based on the diploid phase. When applying such simple models, the following drawbacks appear, when comparing sex to perpetual asexuality:
- Sex breaks up adaptive gene combinations (costs of recombination/segregation).
- Sex halves the genetic contribution of an individual to the next generation (‘twofold cost’ of meiosis).
- Sex halves the chance of a given allele getting into the next generation (cost of meiosis, gene’s eye view).
- For an organism with separate genders, sex may halve the number of grandchildren produced (twofold cost of males).
- The need to locate a mate.
- Time costs.
Sex does have accepted benefits. As Fisher and Muller noted in the 1930’s, sex, largely through recombination, can enable populations to concentrate beneficial alleles and purge detrimental ones independently, and to generate beneficial combined genotypes more rapidly than serial mutation in an asexual lineage. Sexual populations also tend to have more standing variation, which can assist in withstanding environmental change, disease, or parasites. These benefits, however, are at population level. This kind of ‘group selective’, good-of-the-species idea has a problem: how do you get from low frequency to become common enough to reap the group benefit? Especially when, on cost-driven thinking, individuals (or, if you’re a gene-centrist, genes) suffer such a high cost?
The solution (!)
My heretical contention is that the costs are largely illusory: an artefact of perspective and model. Sex doesn’t start with the diploid chicken, but the haploid egg. Regarding diploid somas as the central entity in biology is natural enough. It’s what we are, and is how most eukaryotic organisms spend most of their life cycles, and therefore forms the basis of much of population genetics itself. But in the matter of sex, it’s the wrong start point. Choosing to start with diploidy begs an erroneous view of the transaction. We will never find that elusive benefit for the diploid; there isn’t one. I argue that the entity for whose benefit sex exists is not the diploid organism at all, nor individual genetic loci, but the haploid genomes that nowadays slip almost unnoticed from instance to instance of the larger bodies they often form in diploid partnership, now thoroughly shuffled by the transaction. One might say that haploids don’t exist for the propagation of diploids; it is the other way round.
If we start with fusing haploids, the diploid cell they form is a temporary union, no more an indivisible unit with superior ‘interests’ than a human couple. Despite the superficial elaborations and apparent asymmetries in modern organisms, genetically sex remains a symmetric and mutually beneficial cycle of haploid fusion and division, and looks much the same to haploids as it always did. Diploidy is haploid symbiosis: a marriage of convenience.
While such diploid unions can and do become permanent, they inevitably appear in a background of an established sexual competitor and ecosystem; their repetitive genomes are rarely up to the challenge, while tending to suffer genetic degradation if their paired haploid genomes spend too long in harness. The tools of population genetics, with their inevitable simplifications, tend to obscure rather than illuminate the respective dynamics of the two modes – they overestimate the frequency of asexuals’ occurrence, and over-count their relative production.
The argument should hopefully become clearer if we follow the trajectory of sex from its probable start point: haploid fusion.
The most likely evolutionary sequence is:
- Haploid fusion – sex originated among haploids, not diploids
- Division – return to the haploid state via the ‘back end’ of mitosis
- Independent segregation – gives primitive, coarse recombination
- Speciation – steadily broadens the sexual clade and ecosystem
- Crossover – helps tensioning in meiosis, with far-reaching side-effects
- Multicellularity – nurtures and amplifies paired haploid genomes
- Gender – gamete asymmetry, only possible in multicellular forms.
To start at the very beginning …
1) Haploid Fusion.
The ancestral population in which sex arose, more ancient even than the most recent common ancestor of all modern eukaryotes (being itself sexual), must have been haploid. To get two similar chromosome sets in the first place, they must be copies of a single template in their most recent common ancestor, since duplicated through mitosis in separate haploid lineages. Thus, immediately we can see the that the ‘haploid-centric’ perspective is fundamental, and not merely arbitrary. Diploidy is more usually chosen as the starting point only for reasons of convention and a ‘diplocentric’ bias, rather than evolutionary logic.
There has to be a reason why such fused-haploid cells could prosper of course, but we don’t have to explain everything at once. The following are suggested possibilities for selective advantage, not mutually exclusive:
- Fusion generates an immediate increase in unit size, without going to the trouble of conventional growth. Whether predator or prey, this has the clear potential to be advantageous, to both partners equally.
- It is commonly observed that hybrids exhibit ‘vigour’, being frequently more robust than either parent even if sterile. The cause is related to heterozygosity – deleterious recessives can be masked by their dominant allele, the heterozygote may be ‘fitter’ than either homozygote, and nonoverlapping loci can ‘complement’ each other. There is no reason to suppose that this phenomenon is recent.
2) Division.
Of course, we have an immediate problem – sex as defined is a cycle, alternating haploidy and diploidy. Whatever benefits derive from fusion are discarded on division, yet without a return to the haploid stage, it’s not a sexual system. However, it is not essential that we provide a positive advantage to division. For example:
- Having fused, the cell has been pushed rapidly along its growth phase. If one recalls high school biology, Interphase, where growth and replication take place, is divided into G1, S and G2. Chromosome copies are made during S, so it is certainly possible that a fusion diploid would resemble, to the machinery of mitosis, a normal asexual cell at G2. The cell has grown, and contains approximate chromosome duplicates. Rather than needing a rationale to trigger separation, an early difficulty may have been actually to defer this automated cell division to preserve any benefits of diploidy.
- It is not a given that such early eukaryotes could actually perform mitosis in the diploid state. If they couldn’t fully mitose as diploids, then however beneficial that diploid state may have been, it could only be temporary if such a lineage were to persist.
- Genomes in diploids can suffer attrition from gene conversion events. These occur during recombinational repair, in which one haploid chromosome provides a ‘patch’ to fix breaks in its homologue. In doing so, that region can become homozygous, if it wasn’t already, potentially exposing deleterious recessive alleles to selection, or reducing any benefit derived from complementation at nonoverlapping loci. Additionally these ‘masks’ can themselves increase in number, due to further mutation. Thus, a lineage of haploids which fuses but then never divides may lack evolutionary staying power, ‘selecting for’ the capacity of division as avoidance of a problem rather than exploitation of an advantage.
On both of the latter two points, even if fusion was common and division rare, surviving lineages would be biased in favour of those with the capacity of division. On all three points, no explicit advantage to division itself is suggested. From these early ‘fusers’, it may be the case that only ‘dividers’ have left descendants to the present due to this bias in lineage survival, rather than direct adaptive advantage.
______________________
Intermission 1 – The Basic Transaction.
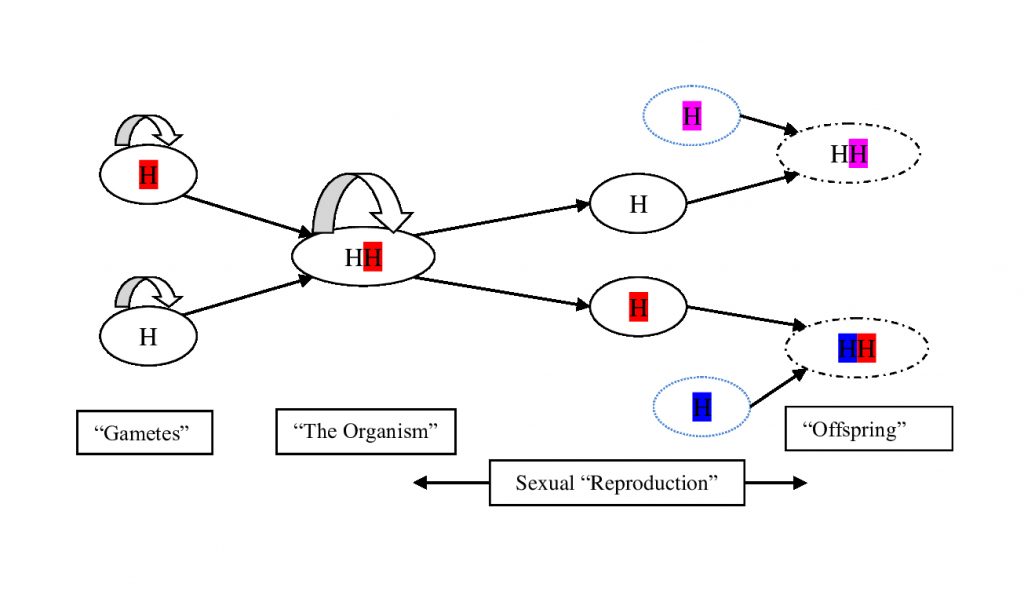
Even with this minimal system, we have all the basic components of modern sex: haploid cells fuse, form a diploid for a period and then reduce back to haploids. At this stage, diploidy need only provide a relatively minor benefit, shared by both haploid genomes, to allow it to compete in a background ecosystem of perpetually asexual haploids. Secondary asexual diploids, meanwhile, always find themselves in direct competition with a parent population already in residence, and will only be ‘average’, on average.
Let’s examine the costs in this primitive system.
Cost of meiosis
The diploid here is nothing more than a pair of haploid genomes sharing a cell, and each haploid is not obviously worse off after the transaction than another parallel pair that remained independent throughout. It is not even necessary that fusion be complete initially – the nuclei may have remained separate, as in Giardia and some fungi today. But either way, one would not view this transaction as a puzzling halving of the diploid’s genetic complement. Any benefits of diploidy accrue to both partners equally; the diploid has no say in the matter. The twofold cost of meiosis is an illusion of perspective.
The costs of meiosis and of males are often conflated. It should be clear that they are in fact distinct, despite taking the same numerical value. John Maynard Smith appears to commit precisely this error in the quote with which I headed ‘The Problem’, though he immediately points out that the cost is not applicable to isogamous organisms – those where both gametes make an equal contribution to the next generation. (Indeed, if sex really did provide a mysterious twofold benefit, as it is commonly assumed it must to cope with males, this stage of isogamy should be an absolute breeze to establish!).
Segregation load.
Segregation load is again a cost viewed from the perspective of the diploid, when comparing a population of perpetual diploids that do not return haploids to similar rivals that do. If a particular diploid combination of alleles is beneficial, sex as depicted may break that beneficial combination due to segregation. However, on this scenario it was sex that brought them together in the first place. An asexual clone will ‘freeze’ and repeat a particular combination, but it simply represents a random draw of two genomes from the wider haploid pool. The sexual population, meanwhile, continues to make random draws. There is no reason to suppose that the asexual’s luck is any better, on the average, than the sexual’s.
There is a rather puzzling assumption implicit in the genetic load arguments, that sex is fine for generating combinations, but should be abandoned the instant it has done so. Yet something better may be just around the corner – there is no reason to prefer ‘stick’ over ‘twist’. If we only count severance of adaptive combinations, without recognising the contribution of the process to their creation, we are guilty of incomplete accounting. As long as net creation exceeds net loss, sex still wins.
Variation
Gradually, as mutations accumulate, the sexual population of diploids may be expected to hold a greater degree of standing variation, even with no recombination, because its genomes circulate in ‘halves’, both uncoupling the diploid and permitting more combinations. This variation may assist local adaptation, and provide a buffer against environmental change – and, indeed, invasion by clonal asexuals.
Ongoing evolution
By circulating as, effectively, ‘half-genome’ fragments (from the diploid perspective), haploids can be tuned more readily by iteration than when locked in harness in a perpetual diploid. A beneficial mutation in the diploid state is interfered with by the other allele at its locus – if recessive, it is not even expressed. The same mutation circulating in bare haploids, however, can increase through drift, then when it begins to encounter copies of itself in diploids, can be further promoted by selection.
Gene conversion
As already noted, homologous repair can expose deleterious recessives to selection. This is an ongoing and growing problem for a perpetual diploid, which exacerbates the problems already mentioned, further diminishing the probability that secondary asexuality will extinguish sexuality. The famous rotifers – ‘an evolutionary scandal’, being a notable exception to the rule that asexual lineages are short-lived – appear to avoid this problem. Their genomes are barely distinguishable as ‘diploid’ at all. If we can’t identify homologues, the repair mechanism is unlikely to do any better. (An afterthought: that may be putting the cart before the horse. The original diploid genome may have been permitted to diverge by suppression of the use of homologues in repair).
In the sexual diploid, conversely, the negative aspects of gene conversion are diminished, since partners don’t stick together long enough for it to become an inconvenience. Additionally, it serves as an incidental mechanism of generating variation, by placing alleles into novel backgrounds.
Networking.
The sexual population of diploids is ‘networked’ by virtue of its haploid vectors. Different solutions to environmental challenges are worked on independently and combined and tested in diploids. Improved versions of the haploid genome can ultimately find themselves shared by every diploid in the future population, a luxury unavailable to the cloned asexual population except by competitive replacement of the entire species.
Asexual diploids
Of course, even at this stage there is nothing in principle to stop the diploid failing to separate, and so forming a diploid asexual lineage. However, such asexual mutants always arise within an established sexual population. A given asexual derivative has committed for better or worse to a single genome out of the myriad of possible variants available to the sexual equivalent. Armed with that single repetitive genome, we are invited to believe that, as a universal principle, this asexual genome would outcompete all variants, throughout a range, in every such contest, if sex is to retain its mystery. This seems a stretch. While the asexual might possess the fittest variant of one genotype (corresponding to a whole chromosome at this pre-crossover stage) it would be unlikely to possess the fittest variant of every single one. The resident sexual has, somewhere, an answer to every competitive challenge the clonal asexual can throw at it, it can evolve more quickly, and within it gene conversion tends to be more a blessing than a curse
In my view, secondary asexuals (those derived from an ancestrally sexual line) are better viewed as a kind of ‘species cancer’ – diploid overproduction which may eliminate the parent ‘body’ in some circumstances, but needs to do so in all to generate a ‘mystery of sex’. Cancer does not cause us to ask ‘Why People?’; likewise, secondary asexuality should not automatically lead us to ask ‘Why Sex?’. Without sex, such presumed diploid threats to it would not even exist, a neat paradox.
______________________
By such marginal degrees, then, a sexual clade may slowly rumble into life, largely untroubled, at this stage at least, by the threat of secondary asexuals, which may briefly flicker but are not up to the task required of them in preserving a ‘mystery of sex’: universal extinction of parent populations.
At what point in subsequent elaboration does the mystery commence? Does sex really have to ‘try harder’ once it discovers multicellular males?
3) Independent segregation.
Eukaryotic sexual recombination generates novel genomes by swapping segments of chromosome in the diploid. Indeed most ‘theories of sex’ are actually theories of recombination. It certainly gives rise to its most far-reaching consequences, yet the active mechanism, crossover, is complex, and its consequences seem to be at population level, requiring certain assumptions both to get it from low to high frequency, and to keep it there. Furthermore, when genetically controlled, a recombining locus may become detached from any benefit it causes.
It is not necessary to evolve something new, however. We have already touched on a mechanism by which limited recombination is achieved as a byproduct of another process: gene conversion in homologous repair. But even with this grossly simplified proto-sexual system, another form of recombination is available ‘for free’: if the haploid chromosome number exceeds 1, we see independent segregation of those multiple chromosomes, shuffling the haploid inputs. The parental haploid chromosomes are not labelled as such; the machinery of segregation simply lines pairs up at random on either side of the metaphase plate and hauls them apart. There is a 50% chance that any given former cell-mates will be separated on division: {A, B} and {a, b} ‘parents’ can produce {A,B}+{a,b} or {A,b}+{a,B} outputs with equal probability. This further increases the variation that the population can sustain.
A chromosomal break can readily drift into a population, if neutral or even if mildly deleterious. While rare, it encounters unbroken versions in meiosis, and there is no independent segregation. But when more common, it will start to encounter copies of itself, and chromosome ‘swaps’ will occur at the break point, purely by chance. As a consequence, beneficial alleles on the one are uncoupled from detrimental alleles on the other, allowing the former to increase in frequency and the latter to decrease, somewhat independently. Additionally, new beneficial combinations can arise through such swaps without the need for serial mutation in one lineage.
Note that this is precisely what Fisher and Muller proposed, but without any necessity for either elaborate mechanism or adaptive benefit to drive its fixation. Such chromosomal breaks form an ‘ideal’ recombining locus, since they do not suffer detachment from any benefit they may promote. They can drift in or out, additionally being sometimes promoted by and sometimes opposed by selection, according to the extent to which the break tends to have net negative or net positive effects. The fragments so formed, meanwhile, can increase or decrease independently of each other. The population is enriched in beneficial alleles, and similarly depleted in detriment, a comparative fine tuning not possible when alleles are chained in indivisible lumps.
______________________
Intermission 2 – On Recombination.
11112222333344445555
An idealised chromosome, each 4-character stretch representing the span of a separate gene.
Cost of recombination
Contrast the above flexible, responsive but accidental mechanism with the assumption underlying the ‘cost of recombination’: that breaking combinations is ‘always bad’. Whether it is or isn’t depends upon current circumstances. Suppose that genes 1111 and 5555 interact – in the jargon, they exhibit epistasis. This means that, in the presence of the other, each experiences either enhanced (positive) or diminished (negative) selection. If a breàk appears between them, potentially swapping 1111 and 5555 into a different background, this could be detrimental if the interaction is positive. However, if the interaction is negative, the break would be favoured.
Additionally, irrespective of interaction, genes may contribute additively to adaptation. If 2222 and 4444 are both locally adapted genes, a break between them may disrupt them, particularly in the face of a stream of less well-adapted alleles migrating in from the wider population. The combined genotype 222233334444 is assumed locally fitter than both 22223333xxxx and xxxx33334444.
In reality, though, there are thousands of genes on a chromosome, thousands times thousands of potential interactions either side of a chromosomal ‘break’, and a great reservoir of combinations to explore available in the wider population. As we saw with segregation load, recombination does not merely break combinations, it creates them in the first place. Migrants don’t merely import locally detrimental alleles; beneficial ones come in on the same tide. Thus whether a particular break point is net detrimental or net beneficial depends upon integration of a great number of variables, which the naive ‘cost of recombination’ serves only to obscure.
Selfish Genes.
The twofold cost is often portrayed from a gene’s perspective, as a halving of its chances to get into any given offspring. Richard Dawkins goes so far as to imagine genes ‘dragged kicking and screaming into the second anaphase of meiosis’ (The Extended Phenotype). His intellectual predecessor John Maynard Smith framed it less dramatically (see ‘The Problem’ above), but both are guilty of exporting their own ‘gene-centric’ conception beyond the boundary of its application.
This needs some unpacking.
By shortening the recombining units involved in the basic transaction, we have dropped down to a new, subgenome level of selection. In a perpetual diploid, the entire paired genome is subject to repeat testing en bloc. But by continuing to reduce to haploids in sex, each chromosome is selected independently, for combinations that work together along its length but in competition with other such chromosomes segregating in the diploid population. Even though we have not yet introduced intrachromosomal segmentation by crossover, we have all the conditions required for ‘selfish genes’.
Dawkins’s catchphrase is frequently misunderstood, in both its parts, though he takes great pains to explain himself. ‘Selfish’ sets up the paradox of his most famous book: how to explain altruism in a competitive, Darwinian framework. His ‘Gene’, meanwhile, is not the molecular biologist’s or the geneticist’s, but a shorthand for a recombinant unit – a stretch of genome bounded by the extent to which it independently recombines. Maynard Smith’s ‘gene A‘ is not a recombinant unit; such things do not exist in asexuals.
Selfish genes integrate into the genomes of the future diploid population; that integration depends entirely upon recombination, and hence upon sex. Along their length, shorter stretches are selected to co-operate, because their futures are linked. While in the diploid state, all genes again have a common interest in co-operation. However, on separation, selfish genes are supposed to oppose that separation. But this is to misunderstand the causal mechanism underlying the metaphor. How can they do so while still remaining selfish genes? While it can be helpful to think in terms of a gene’s ‘wishes’, the metaphor breaks down the moment their means of population integration is removed, and becomes misleading. Paradoxically, a selfish gene cannot ‘wish’ to stop being a selfish gene!
Further, genetic loci themselves are not omnipotent. Most genes supply proteins or RNA tasked with performing some biochemical function or another. This is not readily modified to do something completely different – to ‘plug up the works’ of meiosis in some way. It is simply not mechanically possible for most genes to directly influence their transmission. They must do so through their effects on fitness, in whatever genetic system they find themselves. Indeed genes – the majority – which are at high frequency in the population gain no copies by abandoning sex, since they will generally be homozygous most of the time in any case.
Therefore only a handful of genes have even the notional capacity to effect or benefit from the switch to asexuality. Such genes would leave the sexual population, taking everything else with them – they become de facto a separate species, imprisoning a genome which can compete as a lumpen diploid whole, but whose parts can no longer integrate ‘selfishly’.
This is not to say that mutation to asexuality cannot occur, but it is an error to see this as a genetic competition between genome subunits, when it completely removes the sense in which such subunits have ‘interests’. It is an ecological competition between species.
The only lever available to recombining genetic stretches is to distort their transmission in meiosis, by tinkering with its machinery or attacking their homologue, rather than abandoning meiosis completely. Even here, mechanisms are dissipative. While such drive may distort transmission from an equitable 50/50 for a period, when a distorter becomes common it starts to encounter copies of itself, and either transmission returns to 50/50, or the distorter suffers from attacks upon itself in homozygotes.
Advocates of selfish gene viewpoints sometimes imbue them with a reach that exceeds their grasp. Far from being ‘dragged kicking and screaming’ into meiosis, they file obediently in, as they have always done.
Genetic algorithms
These are computational search heuristics inspired by biological population processes. Digital chromosomes representing varying solutions to a problem are copied and varied further, and the ‘fitness’ of the population members evaluated to determine which persist into the next round. Adding recombination to such programs can have a dramatic effect on search times, and aid escape from local fitness maxima. Recombination is gentler, less ‘speculative’ than mutation, since both parts have already survived in the population.
Even though evolution is not a search as such, the effects of recombination on rate and connectedness in GAs must surely have an analogue in the evolutionary behaviour of the natural populations from which they take their inspiration.
______________________
4) Speciation
So far we have dealt with a single species – the only ‘true species’ on earth under the Biological Species Concept, everything else being an asexual prokaryote or eukaryote. Over time, any persistent reduction in free gene flow around such a population would be expected to lead to divergence between the subpopulations so formed – ultimately to the point at which the subpopulations would be incompatible, due to biological isolating mechanisms. At such a point, the sexual clade would have broadened, and the supposed broad-scale threat of asexuality, to extinguish all parent species, reduced. If there is a probability p that any one species will be eliminated by its secondary asexuals in a given time, with two species the probability they both go is p x p, with 3 species p x p x p and so on. The longer sex survives, the more resilient it becomes, simply by putting its eggs in multiple baskets.
Furthermore, once the growing clade had acquired a degree of ecological divergence, new asexuals would have more than just the resident competing sexual parent species to deal with. The sexual clade may begin to throw up predators, prey, parasites and interspecific competitors, all of which possess greater variation and evolutionary fleet-footedness compared to each new clonal variant. This is termed the Red Queen effect – it takes all your running just to stand still! – and further serves to cement the sexual clade’s position. Secondary asexuals surely arise from time to time, but most either fail to supplant the resident, or succumb to extinction by the several forces ranged against them after replacing the parent species.
As to the original haploid populations from which sex sprang, they too suffer from attrition by the sexual clade. The dynamics provided by sex are not only manifest in the diploid phase. The sexual clade can tune haploid genomes at chromosome level (or lower, given crossover). It can generate beneficial combinations of genes, and possess greater variation even within the haploid phase of its cycle than is available to perpetually asexual haploids. All of these, when added to advantages in the diploid phase, would tend to see gradual extinction of the ancestral asexual haploids.
5) Crossover
Some of the effects of recombination are available passively through independent segregation of chromosomes, discussed above. A distinctive feature of modern sex, however, is further segmentation of chromosomes through crossover. This involves reciprocal swapping of segments of homologous chromosomes during separation in meiosis. A nick is made in one chromosome, the homologue is recruited to provide a bridging patch, just as in repair of accidental breakage, and the resulting interlinked structure is resolved to yield separate chromosomes. There are four different ways to resolve, two of which yield recombinant chromosomes and two return the chromosomes unchanged (but for the patch, which results in gene conversion).
In the ancient repair system from which this derives, the random swap/no-swap result made no difference – the input chromosomes were the identical sisters freshly duplicated during the S phase of mitosis. But with nonidentical homologues from separate parents, and independent futures for the outputs, the consequences are profound.
Because crossover sites are not fixed, it has the effect over multiple generations of exposing yet shorter genetic stretches to independent selection – fully unmasking Dawkins’s ‘selfish genes’, each locus uncoupled from its neighbours and tested independently, over evolutionary time. As with segregational recombination, we get
- Increase of beneficial alleles and decrease of detrimental with reduced interference between loci.
- More rapid creation of novel combinations
- An increase in variation, promoting both local adaptation and evolutionary resilience
These are arguably the most significant consequences of sex, which indeed cause many authors to regard it as the whole ‘point’ of the enterprise; the reason it exists. However, on the argument presented here, this need not be the case. After all, if the gene conversion and segregational recombination above were purely incidental, might it not be the case here too? 50% of crossover sites resolve to a recombinant product, but genes are blind to this. No gene is fundamentally bothered whether it remains linked to the same or to different chromosome-mates, provided the result works.
Population effects are important for lineages, but we do need to get active recombination from low to high frequency before its population consequences can be manifest. Felsenstein (yes, that Felsenstein) considers the case in which that may be mediated initially by drift, subsequently cemented by the resilience of such populations to environmental change. My own preference is to appeal to cellular mechanics. Crossovers assist in the equal tensioning of homologues as they are hauled apart during meiosis. Without them, there is a tendency to damaging asymmetry – one haploid output may lack an entire chromosome, the other having two copies giving potentially damaging trisomies on subsequent fusion. This gives a sufficient reason for crossover to become common in a sexual population without appeal to circumstantial issues such as environmental fluctuation or the extent of negative epistasis in the population. While recombination has significant population effects, which play a substantial role in the success of the clade, those consequences may be arguably a side-effect of its cytological role – an example of what Stephen Jay Gould termed a ‘spandrel‘.
______________________
Intermission 3 – macroevolutionary trends
As a result of these various dynamics, we might expect gradual elimination of the ancestral asexual haploid lineages, possibly complete even prior to our Last Eukaryote Common Ancestor. Certainly, there are no known asexual haploid lineages today.
Since sex has its roots in the prehistory of the entire eukaryote clade, it probably played a significant role in eukaryogenesis itself, that mysterious and probably extended sequence of amendments separating eukaryotes from prokaryotes. We see that as a singularity because we have no surviving intermediates. But sex – serial diploidy – may be at least as important as endosymbiosis, the origin of mitochondria, in the overall sequence. In mitochondria, we are familiar with two genomes in a cell, but in reality, in the diploid phase, there are three.
Subsequent to LECA, the constraints on invasion by asexual diploids and the greater capacity of sexual lineages for anagenesis and cladogenesis would lead us to expect to find a eukaryote clade consisting mostly of sexual forms, with few if any purely asexual haploids, and comparatively few secondarily asexual diploid species. Which is handy, because that’s exactly what we find! All of this was achieved without going anywhere near any ‘twofold cost’. Isogamy can get us a long way.
______________________
6) Multicellularity
Multicellular organisms, featuring multiple tissues in one genetic ‘individual’, have arisen independently a dozen or more times in eukaryotes, but never in prokaryotes. Because the eukaryote clade is built upon sexual roots, there is a close relationship between multicellularity and sex. After all, taking the ‘haploids’-eye-view’, what is a multicellular body other than a vehicle to protect and amplify both genomes, before returning haploid copies in profusion?
Of course there is a little more to it than that.
A multicellular eukaryote forms from either or both of the haploid or the diploid state, according to life cycle, by repeated mitosis of the genome of one or a few cells. The multicellular mode gives advantages of size and of ‘division of labour’ – by differential gene expression, different cell types can perform different functions despite possessing identical genomes. Among those specialised functions is reproduction itself. The cells of a body, or the genes within them, forego their direct reproduction in favour of the reproduction of identical gene copies in the specialised reproductive tissues. Freed of the need to reproduce directly, other tissue cells can concentrate on their own function.
While haploid individuals do occur – for example male social bees – the predominant mode is to form a multicellular soma from the diploid. Even fungal fruiting bodies, which avoid true diploidy until spore formation, have cells that contain both haploid nuclei: dikaryons. Enforcing a reproductive dead-end on diploid cells (or fungal dikaryons) may be easier to orchestrate, since reproduction is performed via production of a non-diploid cell type, a specialism not easily accessed by cells specialised for other functions. (Male bees may be exceptional because they are functionally the sperm of a ‘superorganism’: the hive).
Thus , as a general pattern, the co-operation of all cells in transmission of their shared genome appears to be secured by the specialised exit; opportunities for rogue cell lines to ‘go it alone’ are reduced. Conversely, a hypothetical asexual lineage exploring the first steps towards multicellularity has no equivalent mechanism ensuring intercellular co-operation and specialisation of the reproductive function. Multicellular bodies, I would contend, are an invention of sexual lineages.
7) Gender
The key distinction of ‘male’ vs ‘female’ is relative gamete size, rather than the organs, tissues and bodies with which we are more familiar. Females have the larger gametes, males the smaller. Note that, genetically, there is still little or no distinction between the haploid gametes; it is purely a matter of cellular packaging, with female gametes getting the lion’s share of cytoplasm. For this reason, it is unlikely that gender so defined can arise in unicellular organisms, by asymmetric division; the smaller would suffer disproportionate losses, and any genetic conflict would appear to centre on equal division. By generating male gametes in multicellular organisms from the end of a series of diploid mitoses, however, we get massive amplification of the genome, compensating losses by sheer weight of number. Female gametes, meanwhile, can be nourished, and furnished with additional cytoplasm to kick-start the next generation. Male gametes, being smaller, do most of the dispersing. Being cheaper to produce, they can be generated in greater numbers, but ultimately offspring numbers are limited by females.
______________________
Intermission 4
Cost of Males
Leaving aside, for want of space, the reasons why gamete asymmetry is more stable than isogamy in multicellular organisms, we are finally in a position to examine the ‘twofold cost of males’. Note that this is a long way down the evolutionary trajectory. We have managed to get almost every feature of a modern sexual system, without a whisper of a roadblock from that twofold cost which is supposed to render sex itself a mystery. Even now, it is only an issue for dioecious organisms – those with separate male and female individuals. So the ‘mystery of sex’ resolves to a mere ‘mystery of dioecy’.
Imagine first a multicellular species with slight asymmetry: ‘male’ gametes are fractionally smaller than ‘female’. Fertilisation takes place externally. An asexual offshoot of the female version could, by abandoning fusion, produce asexual diploid offspring directly. But what would such a lineage gain? As in the primitive scenario, all asexual mutations occur against a background of a resident sexual, having variation and faster evolution at its disposal. The asexual is not helped in this conflict by the supposed ‘twofold cost’. We can incrementally increase the cytoplasmic asymmetry, but there is no clear point at which the need for an individual twofold benefit to sex arises. Nor does it arise incrementally, in proportion to the increase in gamete asymmetry. The relative benefit of sex is ‘smeared out’ among the population members, residing in variation already generated, in future capacity for tuning, and in the minimisation of gene conversion-induced homozygous effects.
Finally, we can add resource asymmetry in the embryo to the picture. Females often provide far more to the next generation than males, much more than just a bit of cytoplasm. While males continue to offer nothing more than haploid genome copies, as sperm, spores or pollen, the developing embryo may remain part of the female, as a baby, a maturing fertilised egg or a ripening seed. Half of those babies, eggs or seeds are male. The opportunity finally arises to increase ‘twofold’, by producing only female offspring. This would result in twice as many grandchildren, and exponential increase, compared to the sexual. However, again asexuality happens, when it happens, against a varied sexual background. It is still by no means certain that asexuality should be expected to win this contest a sufficient amount of time to eliminate all parent populations.
The gene’s perspective, again.
Gender is about resource asymmetry, not genetic asymmetry. Most genes reside on autosomes, and as such spend half of their existence in each body, investing equally in two complementary strategies. While in a male, they get inserted into large numbers of mobile gametes, widely dispersed. While in a female, they get inserted into fewer, less mobile but larger and better-provisioned gametes.
Despite several added complexities, from the perspective of any given gene the situation has still not changed from the single-celled state sketched above. In that sketch, individual genes feel no ‘force’ compelling them to remain paired indefinitely – to become asexual diploids. They come together for a period, then part. Even with full recombination, as long as the upstream and downstream companions of any gene constitute a viable haploid, it doesn’t matter whether those companions came from the same or different input haploids. In adding crossover, multicellularity and gender, we have changed nothing in terms of the dynamic between the haploid and diploid states. A given gene in the simple cartoon cycle above will appear in 50% of haploids, deterministically. In the more complex state we are now considering, a gene has exactly the same odds, now offered stochastically.
Haploids go in two by two, haploids come out two by two. Their roles as units are somewhat obscured by their loss of individual integrity as a consequence of the amplification and ‘randomisation’ of the input genomes. With independent segregation and crossover, output haploids are scrambled versions of the inputs – every one of the sometimes billions of outputs is unique; a sexual snowflake. But there is no gene in the haploid which is fundamentally motivated to ‘object’ to this scrambling. Throughout, it remains a genetically symmetrical transaction of pairing and parting.
______________________
Population Genetics.
The ‘twofold cost’, along with other proposed costs of sex, such as recombination and segregation loads, is an argument derived initially from population genetics rather than observation.
Population genetics attempts to model populations in terms of mathematical or computational abstractions – necessarily, simplifications. The population in a simple equation is considered to be panmictic – all individuals are equidistant from all others, and equally likely to mate. Note here that sex is built into the very fabric of the assumptions. The panmictic population is stirred by, among other things, sex itself, through mate search and gamete dispersal. Such populations are also bounded by sex. If an obligate sexual, the range is determined in part by the need for a partner. A male can wander freely, but his genes cannot be passed on if he wanders too far from the available females. The same applies to an unfertilised female.
Now, population genetic models can be applied to asexuals. But there’s a bit of a difficulty there, because they lack both the stirring and bounding effects of sex. We artificially assume they are stirred and bounded, even though a significant cause of these is absent: sex itself. This is OK(ish) until we try and use the same model to compare sex and no-sex. Maynard Smith makes the same error in his textual argument above.
Consider what we’re doing when we use an equation to model a population. In essence, we are putting the population into a massive corral and giving it a good old stir. Individuals can leave the corral – it’s more a line in the sand than a fence – but they can only breed within it. Now, we add a few hypothetical asexuals. But implicitly we keep stirring at the same rate – despite the asexuals not being subject to those ‘stirring’ vectors related to sex. We also keep the same ‘breeding boundary’ – despite the asexuals not being constrained by it.
In a real population in which you bodily moved every new asexual individual to a different location, and kept them corralled, and eliminated all variation in the parent sexuals … then yes, the ‘cost of males’ might be an issue! The simplification is equivalent to metastasis in cancer. If cancer cells were always spread evenly around the body, cancer would kill in far greater numbers.
Because asexual individuals do not disperse in the same way as sexual genes, it is misleading to count their numbers as if they did. If an asexual colony arose within the ‘corral’, partially displacing the resident sexual locally, many of its offspring would be competing not with sexuals, but with other asexuals, reducing the impact of asexuality from that in a maximally-mixed model.
Asexuals are also free to leave the ‘corral’ entirely – here they find no competition with sexuals. Yet the model metaphorically rounds up such individuals and shoves them back inside, efficiently and artificially mixing each such individual to a different location within.
Using a simple model substantially over-counts asexuals.
How easy is it?
A fundamental assumption of ‘cost’ models is that the mutation to asexuality is freely available. This may not be the case. Clearly, to be costly, something must exist that can cash in on the saving. In a land without predators, it costs nothing for a zebra to browse leisurely and alone. Likewise, sex is only costly in the face of an existent asexual.
Firstly, mutation rate is proportional to population size. Small populations are most at risk from asexual invasion, as they lack variation, but equally they are less likely to suffer the mutation(s) in the first place.
Secondly, there is not a uniform susceptibility to the mutation across the tree of life. There are, for example, no known asexual mammals. There may be several reasons for this, but difficulty of mutation may certainly be one. Sperm import imprinted genes which have a profound effect on embryogenesis, while female meiosis is geared towards sperm reception. For a female mammal, it may not be a simple matter of ‘turning off meiosis’. She cannot perform autogamy either, fusing her own gametes, since 3 of the 4 outputs of meiosis are shrivelled ‘polar bodies’. Female mammals, with internal fertilisation, embryo retention and post-partum nurturing might seem to have the most to gain from asexuality, but they may also, as a group, find that amendment the hardest to achieve. This, rather than cryptic twofold benefit, may account for its absence.
I am only aware of one asexual bird, the turkey. And those offspring are all male, and so can’t form a lineage.
Asexuality becomes commoner as we go through the reptiles, and on to fish. However, even there, many of the examples arise from a process known as hybridogenesis rather than mutation. A significant competitive difficulty facing hybridogenesis is the replacement of both parental species, if they are ecologically distinct. Therefore, such mechanisms don’t pose a great threat to sex on the grand scale.
______________________
To summarise, sex is woven into the fabric of the whole of eukaryote biology. It is an error to view asexuality as if it were a simple adaptation for diploids; much of the ongoing puzzlement derives from adherence to that standpoint. Contingency, side-effect, central stability and exaptation play at least as great a part as adaptation in the prevalence of sex, while the expectation that a twofold benefit is still required is an artefact of the diploid stance in oversimplified models. Sex and diploidy are fundamentally an adaptation for haploids, with far-reaching consequences.

“7) Gender
The key distinction of ‘male’ vs ‘female’ is relative gamete size, rather than the organs, tissues and bodies with which we are more familiar. Females have the larger gametes, males the smaller. Note that, genetically, there is still little or no distinction between the haploid gametes; it is purely a matter of cellular packaging, with female gametes getting the lion’s share of cytoplasm. For this reason, it is unlikely that gender so defined can arise in unicellular organisms, by asymmetric division; the smaller would suffer disproportionate losses.
I have a distinct feeling that details, and a lot of them, have been missed here…
I hope they exist…
“By generating male gametes in multicellular organisms from the end of a series of diploid mitoses, however, we get massive amplification of the genome, compensating losses by sheer weight of number. Female gametes, meanwhile, can be nourished, and furnished with additional cytoplasm to kick-start the next generation. Male gametes, being smaller, do most of the dispersing. Being cheaper to produce, they can be generated in greater numbers, but ultimately offspring numbers are limited by females.”
This sound more like philosophical speculations than scientific… I think I know why… It’s wishful thinking rather than existing evidence…:-)
Allan, are you going to publish an abridged version of this as a book?
He should, but when is he going to correct the mistakes and unfounded assumptions? Now? Or after? 😉
Allan, a few quick reactions.
1. Basically, your arguments are correct. I have long argued that in isogamous species (no difference in size between female and male gametes) the twofold cost of “sex” is not present. That corresponds to your haploid model. (By the way, the question is not about behaviors of sexes but about outcrossing-with-recombination. But the word “sex” attracts more attention, so lots of people use it).
2. Once female gametes become big and expensive, the Carl Duesing / R.A. Fisher sex ratio argument predicts evolution toward equal resource expenditure in females and males, and then we start to have the twofold cost of sex.
3. Shozo Yokoyama and I in 1976 computer-simulated cases that can be made to model your haploid model. See here. We found that within-population selection did predict an advantage to recombination. (Don’t be too put off by the description of heterozygotes for the recombination locus showing dominance or recessiveness — in that model the selection is basically on haploids, in effect. Recessiveness just corresponds to the asexual haploids not being able to recombine with the others.)
What if they are not?
I can think of at least one, the usual suspect, that saves all evolutionary inadequacies, shortcomings and the wildest speculations…
Wait for it…
.
.
.
Natural selection! Yahoo! 😉
ETA: I’m soooo glad I’ve decided to take time off coz this OP is going to be the assembly of the wildest speculations in the world…without a shred of evidence…
Speculation Genetics will dominate, as usual…
And I was wondering why Vincent Torley had changed his name to “Allan Miller”.
😃
Indeed, touché! 🤣 But, it is an abridged version of the 50 page thesis I previously offered! I haven’t counted lately, but it came in at 6500 words while it was still in Word, about 0.65 VJTs in length!
The problem is: what to leave out? We need explanations for fusion and division, recombination, wide distribution, and asymmetries: the role of multicellularity and gender. And, I wanted to critique misapplied selfish gene thinking, overcounting in population genetic models, and the ‘cost-based’ approach itself.
There isn’t a simple ‘back-of-a-cigarette-packet’ explanation for sex. In fact the expectation that there should be is one of my targets. Its dynamics, and relation to asexuality, are detailed and involved.
J-Mac,
Funnily enough, I have made very little appeal to natural selection in the piece. I am critical of an overly adaptationist stance – and there is a substantial subtlety that I know will pass you by relating to the effect of sex/asex on selection.
That’s great!
But, I’m not sure if you realize that, but you just took away one, and the only “weapon”, Joe uses when his math in population genetics doesn’t work, which is always… 😉
I have a distinct feeling that natural selection will make its reemergence in its one form, or all of them, the all-capable one…
I wish there were ways to ignore the whole OP and all the comments on it… 🙁
Sex – a matter of perspective?
Sex – a matter of bias, clearly!
But there is! Just stop reading and commenting at TSZ altogether.
One aspect of my viewpoint that I think bears emphasis is that recombination can occur without crossover, through independent segregation. It occurs as soon as a fusion/division cycle initiates, if the haploid chromosome number exceeds 1, and hence needs no more reason than that to realise some of its effects.
The other aspect is that, from the perspective of the haploid genome, modern sex is hardly any different from isogamous sex.
Yes, though there is an intermediate step of multicellularity. It is not likely that a single cell can be divided asymmetrically, whereas multicellular somas can either generate male gametes in profusion, or somewhat fewer of the more expensive female ones (or both, of course). You get sex ratio stability in dioecy, and limitation by females. But where I have always struggled is in accepting that this generates a ‘twofold cost of sex’ (rather than, say, dioecy, and even then I’m a bit of an outlier).
If there isn’t a ‘twofold cost of meiosis’ (see isogamy), and the genetic competition is notionally ‘isogamous’ even if cytoplasmic contribution isn’t, where does the ‘cost’ reside? I know that asexual ‘females’ can double their production of grandchildren, but it’s a strictly ecological competition between species, not a genetic one within. And these contests only take place when the mutation occurs. I feel the cost-based approach raises a false expectation: a ‘twofold benefit’. I don’t think that is necessary.
Crikey, can’t help you there! If only there were some mechanism. God helps those who help themselves, I guess …
Very much so – though not in the way you mean. There is a diploid bias in much analysis. I’m trying to argue for turning that on its head, to look at it from the ‘inside out’. I’m criticising the mainstream! And Dawkins. You should be pleased.
Allan Miller,
I appreciate the effort you put into this discussion. The area of interest for me is recombination as it appears to be a real change mechanism. Is there any evidence that this process can create new genes in animals. Art Hunt shows evidence that it could create a new gene in corn.
Boy! Let Art Hunt know to tell my kids something they don’t know…
Evidence of what, Bill?
How do you determine and new gene function?
By assuming, like Hunt did?
It’s a hell of the evidence…
I can to the opposite and there is nothing Hunt can to prove me wrong, because all he has is speculative assumptions…
Don’t be so gullible, Bill!
I was hoping you’d present some evidence I could take apart…
You can’t even defend your fairy tales… You should be writing fables for kids… you know? 😉
And let you, Joe and Allan run wild with your speculations and call it science?
No way! 😉
J-Mac,
Have you seen his paper? I had Behe review it and comment.
Behe said the gene had a new function???
Sorry but I have not seen it but I’m almost positive the gene is either broken or dysfunctional to some degree… 😉
Got the link?
I gotta a feeling Bill, you can’t find the paper and the comments, so I’ve found something that relates to your supposed claim…
Let me know if it is related…
I sent you an email.
ciao!
J-Mac,
Thanks for the paper it is exactly where we need to go. Behe’s comments paralleled the paper.
Only to those who like to watch.
I’ll get me coat…
At last!!!! 👍
Judging from the amount of flack, you must be over a target.
There are numerous different forms of recombination. The one directly associated with sex doesn’t tend to produce ‘new genes’, but new genotypes – genes are swapped into novel genetic backgrounds. Crossover can take place mid-gene, and hence create chimeras, but the inputs are already alleles.
The kind of recombination that moves fragments about is more likely to have a hand in ‘new genes’ (how defined?), but that isn’t particularly a feature of sex.
(Eta – crossover can cause segment deletion, duplication or inversion, however, which can be a significant motor of change).
Allan Miller,
I am slowly munching my way through your opus magnum, but will be dropping some comments to keep the momentum. For starters, there is this one:
[citation needed]
As discussed previously, the main argument in support of higher extinction rates of eukaryotic asexual lineages is their relative youthfulness, visible as a “twiggy” phylogenetic distribution. However, there are several plausible alternative explanations for this pattern, such as low transition rates to asexuality. Hence, I would like to see you support this statement. What makes you say that most secondary asexuals fail to supplant the resident or go extinct after doing so?
Well, if that is your concern, you should put in a little more effort. So far you have only posted a lot of comments with the word “speculations” in it. I haven´t seen you raise any substantial objections yet.
I did notice that you are cutting down on your !!!lol!!!s. Very good. Keep it up!
A. Miller, TSZ Press 2020 😉
No, there are mechanistic arguments too.
Gene conversion – it exposes deleterious recessives, and with added mutation more will be generated.
Competition – a varied resident must have an ‘edge’ over a clonal invader, resisting replacement.
Broader Ecology – a varied and evolvable ecosystem will be a greater challenge to a clonal species.
Note that at the point I make this claim, I’m talking about the primitive, minimal system, in single-celled organisms. I discuss low transition towards the end – in birds and mammals asexuality’s pretty much non-existent, probably due to the much greater complexities of achieving it in (those) multicellular forms. But I do wish to concede to the ‘cost’ brigade that their assumption of freely-available asexual competitors may be valid in early evolution, since stopping division is not in itself difficult.
Corneel,
… and, as I noted, at least one of your room-mate’s models must depend upon competition with the parent sexual. If they tend to be found in more northerly latitudes, or higher altitudes, and hence are subject to greater extinction by marginal habitat, what pushes them there, if not direct competition where sexuals are in residence?
Allan Miller,
‘ere we go – first hit on ‘gene conversion asexual’. Although to my chagrin it finds the opposite effect for the particular thing I was looking for! But it does demonstrate reduced polymorphism and selection interference.
This, however, shows clear loss of heterozygosity through gene conversion. And, incidentally, is another example of hybridogenesis, a mode that would find it hard to displace both parent species.
So? What was Behe’s conclusion exactly about the “new gene function’?
Mechanistic arguments go both ways: You yourself mentioned the break-up of adaptive gene combinations and mate limitation as downsides of sexual reproduction. So which arguments carry more weight?
Making it even harder to validate your claim. Whether asexuals experience inherently higher extinction rates remains an undecided matter.
Asexuals thrive in a habitat where sexuals cannot survive, and you claim this as a benefit of sexual reproduction? That’s a good trick.
Heh, and as it happens, the senior author on that paper is my former roommate. Well done! I note that, despite the higher rate of deleterious mutation accumulation in asexuals, no fitness difference is apparent yet between sexual and asexual Timema stick insects:
So it appears, and the process of deterioration is extremely rapid in this system, resulting in a rapid turnover of asexual lineages. There is something very peculiar going on in those hybrid genomes, so I am not convinced this is a very representative example.
Now, if you wish for me to ever finish your OP, stop burying me with publications 😉
Into what? Pretending that everything is ok, like you do?
There has to be something substantial to object to first, just in case you didn’t realize that… I’ve already objected to the evolutionary nourishment and bathing mechanism, but Allan ignored it, as he should, if he wants the fairy tale to go on in the oblivion… 😉
Are you kids playing with your daddy’s computer again? Shoo!!
As I say in the OP, I think ‘breakup of adaptive gene combinations’ is overplayed and an incomplete view of the dynamic. If we’re talking local adaptations, this is a local problem, hardly troublesome to the sexual population as a whole, and anyway counterbalanced by the positive input: networking of beneficial alleles segregating in the wider population.
Mate limitation isn’t a ‘cost’ if there are no asexuals around. You can’t compare costs if there are no real alternatives, only hypothetical ones. What’s the cost of not having a titanium skull? 😃
However, the situations aren’t symmetrical. When an asexual arises, there is always a sexual around.
And all others! But we can look to protists for clues.
They must, from basic principles. If a new allele arises in a population, it is much more likely lost than fixed (I’m well aware that’s not quite what you mean!). But you seem to be rejecting the tradition going back through Fisher and Muller to Weissmann, that variation is a good thing, which sex provides and asex doesn’t. With colocated close competitors, I don’t see why we would expect the comparatively invariant ones to win these contests, generally, regardless of other factors.
No, it demonstrates the failure of asexuals to compete where ranges overlap.
“Citation required”.
Citation given.
“Enough with the citations!” 🤣
J-Mac,
I couldn’t work out what you were trying to say. You bolded a couple of bits about female gametes having the larger amount of cytoplasm (definitionally true), then trailed off a bit …
J-Mac,
That cells could modify genes by deterministic processes like recombination.
It’s not that deterministic. Crossover siting is stochastic (though not ‘random’), and the genomes swapped need bringing together. To achieve a given swap in you, say, something would have to have arranged for your mum and dad to meet, deterministically.
That hardly constitutes even an assumption for a new, functional gene…
Of course not!
Nobody knows what you wrote means, including you, or the sources you copied it from…
That constitutes 99.9% of the evolutionary blather…just in case you didn’t know…
Great stuff! Thank you, Allan.
I don’t think that there is a mechanistic barrier to asymmetric cell division, which sets me to ponder on the observed correlation between anisogamy and multicellularity.
There’s a pile of stuff on volvocine algae I need to learn about.
It’s designed, obviously…
Obviously a lot of nonsense that I will not even bother to dissect. Not just in the OP, but also from the confused fitness gurus loitering this place. But at least you’re dropping the “descent with modification” craziness. Kudos to you for that.
Allan Miller,
The process is not a random process. With high levels of determinism. Stochastic seems like a reasonable definition.
The mechanistic barrier I was thinking of resides in the survival of the resultant gametes, rather than the division process per se – although it’s hard to see how haploid genomes would permit one to hold on to more than the other. I like to see haploid conflict in the diploid as ‘arm-wrestling between partners of approximately equal strength’. It tends towards symmetry, which is one reason sex is so stable in the long run, despite Selfish Gene assumptions.
Hmmm. ‘High levels of determinism’ is a bit of an odd concept, to me. There’s a bias in crossover siting – it concentrates in ‘hotspots’. If one sees that bias as ‘a bit deterministic’, that’s fair enough I suppose – but even then, the haploid inputs to the process are pretty random in any given instance, as is the 50/50 coin-toss chance that any given crossover gives a recombinant output.
OK, thanks for dropping by and letting me know.
It’s still ‘descent with modification’ though. It’s just that, with sex, the units subject to that descent/modification process are below the level of the entire genomes in asexual lineages.
I do my best. But your own contributions are under your control, so if you had a point, I’m afraid I missed it.
No, I didn’t, thanks! I certainly labour under the illusion that I understand this stuff, but I guess it’s like you and the word ‘quantum’ …